CD Spectrometry for Protein Structure Determination
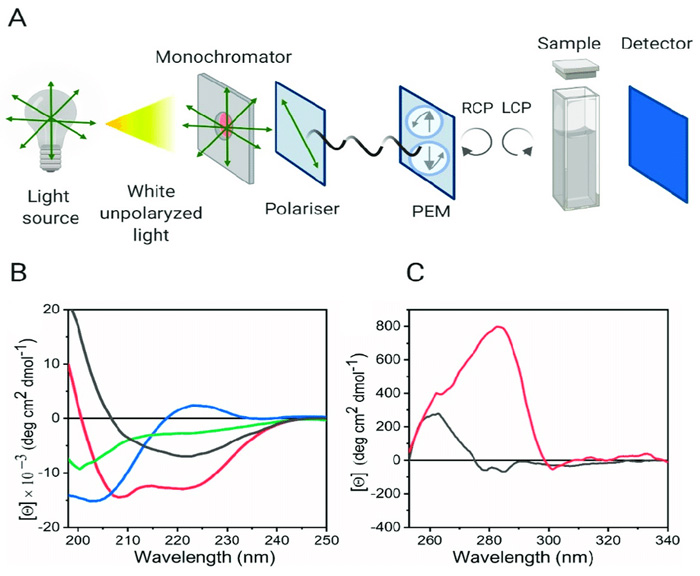
Fig.1 (A) Schematic representation of the Circular Dichroism instrument configuration. (B) Representative Far-UV CD spectra of the characteristic secondary structure of proteins and peptides. (C) Near-UV-CD spectra of two different proteins. (Pignataro, Mm. F.; et al. 2020)
Introduction to Circular Dichroism (CD)
Circular Dichroism (CD) spectroscopy is widely used to study large biological molecules. It is a measurement of differential absorption of left- and right-handed circularly polarized light. CD spectra in the far-UV region accurately measures changes in the secondary structure of a protein, while in the near-UV region it gives detailed information of tertiary structure changes. CD spectroscopy serves as a powerful tool for rapid determination of protein structure and assessing the physical stability of proteins requiring a minimal amount of sample compared to other conventional techniques.
Circular Dichroism (CD) Services
BOC Sciences applies CD spectroscopy for diverse applications including monitoring conformational transitions, investigating the thermodynamics of unfolding and protein-ligand interactions, studying the secondary structure of proteins, etc.
We provide following services to support your discovery programs:
Characterization of protein structures
- Determine the secondary structure of proteins that cannot be crystallized.
- Investigate the effect of drug binding on protein secondary structure.
- Study the effects of environment on protein structure.
Dynamics studies
Observe dynamic processes, e.g. protein folding and fold recognition.
Conformation studies
- Collect the complementary information about the secondary and tertiary structural state of a protein for protein conformation.
- Study the ligand-induced conformational changes.
Binding detection
Investigate the protein-protein and protein-nucleic acid interactions.
Our Advantages of Circular Dichroism Services
- We have established a CD spectroscopy method with the system which can accommodate two 96-well plates (up to 192 samples) enable its usage for high-throughput screening.
- Our team are confident in performing high-quality CD spectroscopy by conducting detailed, sensitive and accurate detection with much smaller amounts of both proteins and drug candidates to be used in the screening.
- We also provide professional and reliable data analysis with high sensitivity, accuracy, and resolution to meet your specific needs of your project.
Equipment
JASCO J-1500 Circular Dichroism Spectrophotometer
Reference
- Pignataro, Mm. F.; et al. Evaluation of Peptide/Protein Self-Assembly and Aggregation by Spectroscopic Methods. Molecules. 2020, 25(20): 4854.
※ It should be noted that our service is only used for research.

One-stop
Drug Discovery Services
- Experienced and qualified scientists functioning as project managers or study director
- Independent quality unit assuring regulatory compliance
- Methods validated per ICH GLP/GMP guidelines
- Rigorous sample tracking and handling procedures to prevent mistakes
- Controlled laboratory environment to prevent a whole new level of success
Online Inquiry

