Fragment-based Screening Methodologies
Once the fragment library is established, the most critical step is to screen and identify active fragments that bind weakly to the target protein. Various biophysical techniques have been applied in the fragment screening for identification of fragment compounds. These different screening methods can offer a great number of information on the ligand, providing important guidance on the following hit fragment optimization and connection.
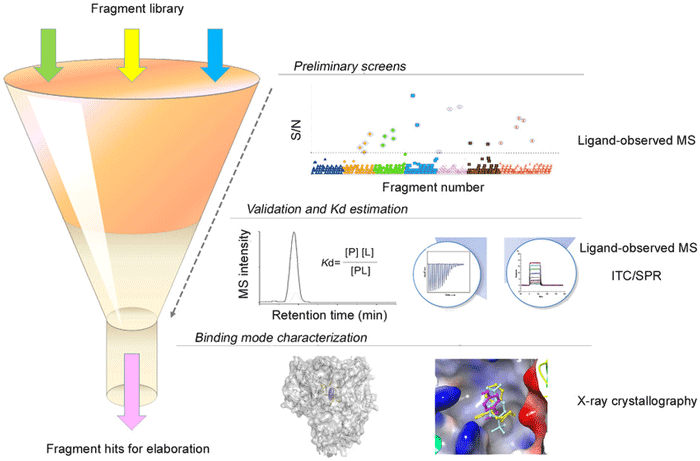 Fig.1 A fragment screening process incorporating different biophysical assays for efficient fragment hit identification. (Chen, X.; et al. 2015)
Fig.1 A fragment screening process incorporating different biophysical assays for efficient fragment hit identification. (Chen, X.; et al. 2015)Our Services of Fragment-based Screening Methodologies
BOC Sciences utilizes comprehensive fragment screening strategies to provide rapid and accurate compound characterization and targeted-ligand binding mode analysis for our customers.Our fragment-based screening platform has equipped with diverse state-of-the-art facilities and well-trained operators to perform your fragment-based screening projects.
X-ray crystallography
X-ray crystallography is a powerful tool in structural biology to determine the structure of biological macromolecules and proteins. Our teams in BOC Sciences can use this technology to help you better understand the protein-fragment interactions and offer guidance on the optimization of the lead compound
Differential scanning calorimetry
Differential scanning calorimetry is validated and applied as a fast and inexpensive screening approach for the thermal unfolding studies of target proteins. We use this valuable tool to characterize the thermal stability of protein biopharmaceuticals and evaluate potential drug candidates to advance into development.
Surface plasmon resonance
Surface plasmon resonance spectrometry is a time-resolved measurement of target-ligand interactions and available for supporting the analysis of fragments. With the application of SPR spectrometry technique, we can study the analogues of seed fragments, determine their structure-activity relationships and binding kinetics.
Thermal shift assay
We adopt thermal shift assay to quantify the variation in thermal denaturation temperature of protein in different surroundings and identify target fragments by monitoring changes in protein stability. We also provide services for determining and optimizing a variety of conditions such as mutations, salts, pH, and oxidation/reduction.
Microscale thermophoresis (MST)
Microscale thermophoresis makes use of the intrinsic fluorescence of proteins to quantify the binding affinities of ligands and determine the binding sites. Our MST-based fragment screening services imply the in-solution steady-state binding affinity (KD) and other important information about fragment-induced target aggregation or precipitation for each fragment.
Isothermal titration calorimetry (ITC)
Isothermal titration calorimetry method provides an insight into the enthalpy changes rather than rely on entropic effects, which assist in subsequent fragment optimization. We use ITC approach to directly determine the thermodynamic parameters including the enthalpy (ΔH) as well as the overall free energy (ΔG) of affinity for the binary system and measure the weak binding of hits.
Nuclear magnetic resonance (NMR) spectrometry
As a most commonly used method in fragment screening, nuclear magnetic resonance can measure low-affinity ligands with high sensitivity and allows for the analysis of fragments. Our NMR groups are capable of measuring binding affinities for even weak ligands and delivering structural information of the binding site by performing both target-based NMR screening and ligand-detected NMR method.
Mass spectrometry
Mass spectrometry is able to directly visualize complex formation with little amounts of label-free sample required. BOC Sciences has developed powerful MS platforms aiming to offer diverse mass spectrometry methods including liquid chromatography mass spectrometry (LC-MS), high-performance liquid chromatography-mass spectrometry (HPLC-MS), capillary electrophoresis-mass spectrometry(CE-MS), gas chromatography-mass spectrometry (GC-MS) and affinity selection-mass spectrometry (AS-MS) for observing protein-small molecule complexes and analyzing the protein-ligand interactions.
Bio-layer interferometry (BLI)
Fragment-based screening with the application of BLI plays a role in drug discovery. We use bio-layer interferometry to perform the measurement of interaction of a fragment with a protein target on its biosensor and kinetic constants are derived from binding data, helping to decrease false-positive rates in hit confirmation.
These approaches offer a comprehensive data package that can increase confidence in the selection of hits for the following optimization phase.
Our Advantages of Fragment-based Screening Methodologies
- We carry out fragment screening that provides desired throughput and necessary robustness to meet the high fragment concentrations, ensuring your efficient and high-quality fragment-based drug discovery.
- With professional expertise and experience in biophysical techniques, our teams can conduct fragment screening campaigns by combining different screening approaches and therefore reduce the limitations of a single technology.
- Our scientists are capable of interpreting data obtained from these biophysical screening approaches and offering valuable suggestions that assist in the selection of hits for the following optimization phase.
Reference
- Chen, X.; et al. A Ligand-observed Mass Spectrometry Approach Integrated into the Fragment Based Lead Discovery Pipeline. Srep. 2015, 5: 8361.
※ It should be noted that our service is only used for research.

One-stop
Drug Discovery Services
- Experienced and qualified scientists functioning as project managers or study director
- Independent quality unit assuring regulatory compliance
- Methods validated per ICH GLP/GMP guidelines
- Rigorous sample tracking and handling procedures to prevent mistakes
- Controlled laboratory environment to prevent a whole new level of success
Online Inquiry

