GC-MS for Target Characterization
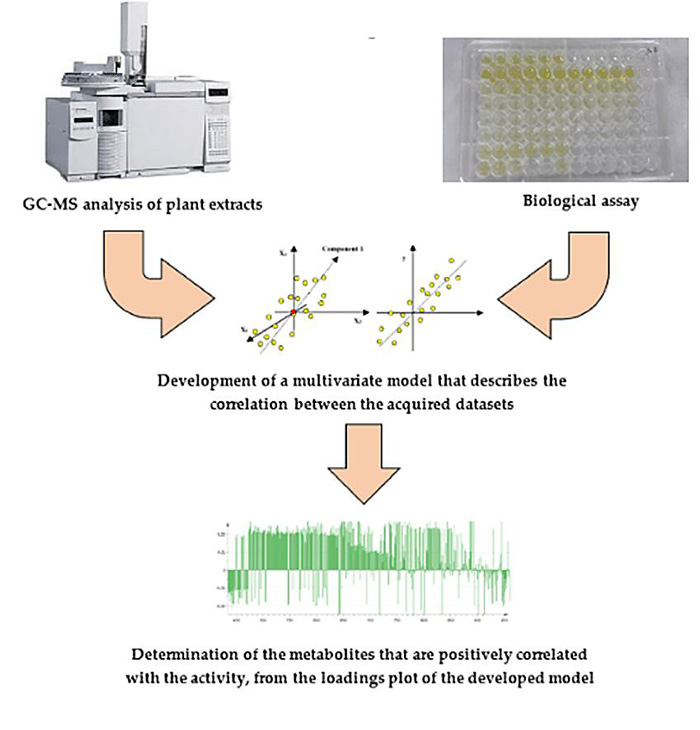
Fig.1 Typical procedure of the gas chromatography–mass spectrometry for characteriziation of the active plant metabolites. (Nokhala, A.; et al. 2020)
Gas chromatography-mass spectrometry technology is an analytical approach used for the separation and analysis of samples that are able to be vaporized without thermal decomposition. It is one of the most frequently applied analytical techniques for both quality and quantity chemical.
Advantages of GC-MS
- GC-MS process is presented that allows to identify components in a liquid mixture and enable to determine their relative concentration.
- Simultaneous and highly specific detection for screening as well as confirmation of drug candidates can be conducted in GC-MS procedure in one single step.
Process of GC-MS
The sample is firstly mixed with a solvent and then injected into the gas chromatography, the sample solution can be vaporized into the gas phase quickly.The separation of components in GC-MS is carried out based on differences in behavior between a flowing mobile gas phase and a stationary liquid phase.
GC-MS Services
We have established multiple mass spectrometry approaches in BOC Sciences for high sensitive and selective screening as well as utilize them in a diversity of applications to support your drug discovery projects.
- Our team has established GC-MS instruments and equipment to separate, quantify and identify different mixtures of small and volatile compounds.
- We also provide residual solvent measurement for purity analysis for synthesis of a drug candidate before the follow-up toxicity testing, helping to improve efficiency in the early stage of drug discovery.
Equipment
Shimadzu GCMS-QP2020
Reference
- Nokhala, A.; et al. Investigation of α-Glucosidase Inhibitory Metabolites from Tetracera scandens Leaves by GC–MS Metabolite Profiling and Docking Studies. Biomolecules. 2020, 10: 287.
※ It should be noted that our service is only used for research.

One-stop
Drug Discovery Services
- Experienced and qualified scientists functioning as project managers or study director
- Independent quality unit assuring regulatory compliance
- Methods validated per ICH GLP/GMP guidelines
- Rigorous sample tracking and handling procedures to prevent mistakes
- Controlled laboratory environment to prevent a whole new level of success
Online Inquiry

