HTS Assay Development
The main goal of assay development is to adapt and validate the existed assay technologies to meet the specific needs of a project. These assay methods therefore enable to perform a proper measurement through the assay optimization efforts. Major considerations in assay development include: relevance, robustness, reliability, practicality/feasibility, cost and automation. Specific capabilities of assay development include multiple assay methods with various read-outs, optimization and validation for primary and secondary assays.
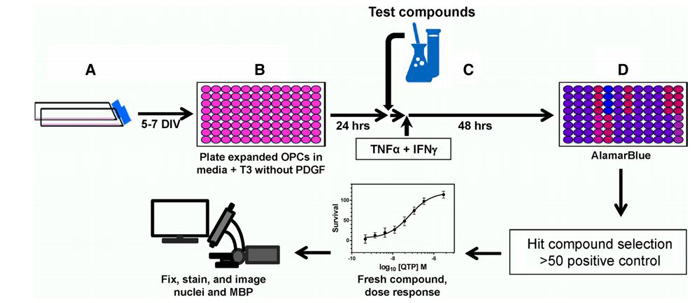
Fig.1 Schematic for high-throughput screening assay of VP35 function. (Luthra, P.; et al., 2017)
Our Services of HTS Assay Development
We offer a wide range of assays for diverse purpose including biophysical assays, biochemical assays, cell-based assays, primary and secondary assays and quantitative High-Throughput Screening. All of them are optimized and available for the comprehensive assessment of your targets for potency, efficacy, kinetics, selectivity,etc.
Biochemical assays
Biochemical assays are a series of commonly used procedure to help in understanding the functions of biomolecules and characterization of targets. We offer a diversity of biochemical assays for qualitative or quantitative assessment of various biochemical processing pathways or the activity of biomolecules of your interest. Here we perform enzymatic assays, binding assays and higher throughput screening to meet your drug discovery needs.
Cellular assays
Cellular assays is a important functional/phenotypic tool for selecting promising lead compounds candidates. They are also available for high-throughput screening thousands of compounds under different conditions due to the unique feature of their amenability to miniaturization and multiplexing. Nowadays, cell-based assays are applied in studying the biological activity, biochemical mechanisms and off-target interactions. BOC Sciences have experiences and expertise in conducting different types of assays such as reporter gene, second messenger and cell proliferation, etc.We also have multiple cellular readouts and cell models to achieve the best outcome.
Biophysical assays
Biophysical assays is a valuable component of drug discovery projects. Scientists can understand the drug-target interaction on a molecular level with the application of them. Biophysical assays offer affinity and kinetic data such as, association rate constant (Ka), dissociation rate constant (Kd), dissociation constant (KD), entropy/enthalpy change which are crucial information for the selection of analytes with the most promising properties.Our biophysics teams have a set of biophysical tools for determination of structure, binding kinetics, affinity, specificity and thermodynamics. We can also provide a comprehensive biophysical assessment of your compounds and deliver the hit findings for Fragment-based lead discovery.
Primary and secondary assays
We assess and validate key assay parameters in primary and secondary assays experiments to deliver qualified assays for subsequent drug screening.
- Relevance: coefficient variation of controls and correlation of replicates between plates with a small set of compounds
- Robustness:validate parameters(Z-factor and CV-factor)and controls (positive, negative, and neutral)
- Reliability:tolerance of effects from DMSO and systematic effects (e.g., plating artifacts, liquid handling errors, etc.)
- Feasibility: assays with less than three liquid additions and are endpoint.
Quantitative High-Throughput Screening (qHTS)
qHTS is a process in which each compound of a large chemical library will be tested at multiple level of concentrations. BOC Sciences experts use it to combine the pharmacologic dose-response relationship with the speed and accuracy of automated HTS. The qHTS technology therefore provide the most detailed and convincing information of how validated assay affect HTS results. Our qHTS process is as follows:
Automated robotic screening(384 and 1,536-well format)
Plate incubation and chemical library storage
Pilot screening(1.5 million compounds per day approximately)
Miniaturization of assay volumes(from 2 µL to 6 µL )
Data processing, curve-fitting and classification through an informatics pipeline
Generation of pharmacological actives to increase the reliability and reduces false-positives and -negatives)
Our Advantages of HTS Assay Development Services
- Our experts execute strictly several standard guiding principles when developing off-the-shelf or tailored assays for our customs.
- We have professional knowledge in identifying the most suitable biological model and assay technology adapted to improve the efficacy of your project.
- BOC Sciences also provide services of thoroughly validation of the assay and give guidance on the optimization of follow-up experiments to ensure the best experimental conditions for your assays.
- We can help to assess the compatibility of the target assay with technologies and high-throughput screening by carrying out evaluation regarding the relevance of biological assays, automation and cost-effectiveness.
Reference
- Luthra, P.; et al. Topoisomerase II Inhibitors Induce DNA Damage-Dependent Interferon Responses Circumventing Ebola Virus Immune Evasion. mBio. 2017, 8(2): e00368-17.
※ It should be noted that our service is only used for research.

One-stop
Drug Discovery Services
- Experienced and qualified scientists functioning as project managers or study director
- Independent quality unit assuring regulatory compliance
- Methods validated per ICH GLP/GMP guidelines
- Rigorous sample tracking and handling procedures to prevent mistakes
- Controlled laboratory environment to prevent a whole new level of success
Online Inquiry

