SPR Spectrometrys for Structure Determination
What is Surface Plasmon Resonance (SPR)Spectrometry?
Surface plasmon resonance spectrometry is an optical technique used to measure molecular interactions based on biosensor chip and have already been widely used in drug discovery. In the SPR, target protein is attached to the sensor and analyte (small molecules or proteins) are used to flow over its surfaces and a binding interaction is able to detected using an optical method that measures small changes in refractive index. Thus, multiple binding parameters including kinetic, stoichiometry and thermodynamic are able to be measured by SPR.
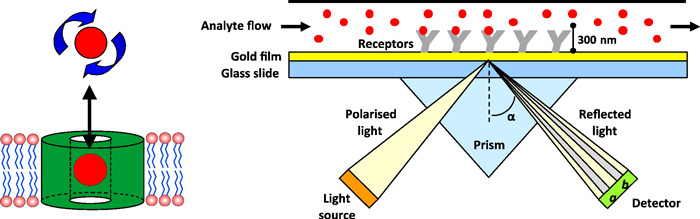
Fig.1 The basic instrument set up for an SPR biosensor. (Patching, S. G. 2014)
SPR in Drug Discovery
SPR gives crucial information for hit confirmation, lead characterization and optimization including:
- Specificity of binding between two molecules.
- Concentration of target molecules.
- Kinetic parameters of binding and dissociation.
- Bond strength.
Advantages of SPR
- Articles under measurement need not be labelled.
- Suitable for a variety of solutions (turbid, opaque or colored).
- Enable to monitor the dynamic process of reaction in real time and continuously.
- Convenient and fast detection.
- Wide range of application.
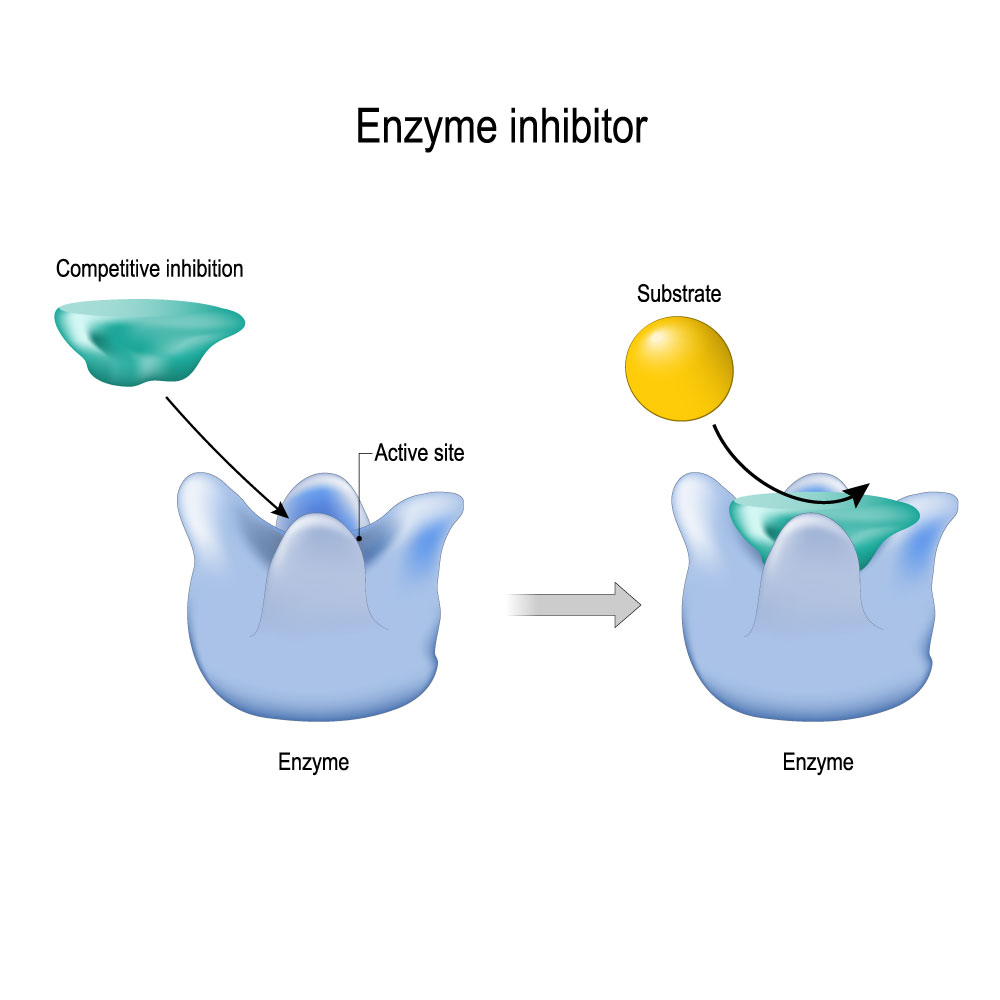
Fig.2 SPR biosensor can be applied to monitor the binding events of enzyme inhibitor and enzyme.
Our services of SPR
BOC Sciences has already applied this sensitive method in high throughput screening to provide more valuable data and information for our customers.
Fragment-based screening
- The detection and characterization of fragment binding are facilitated by sensitive biophysical techniques which are capable of detecting low affinity interactions of low molecular weight compounds.
- Due to its low protein consumption and ‘label-free’ methodology, scientists from BOC Sciences use SPR to screen low molecular weight ‘fragment’ libraries and obtain a good deal of SAR information from the primary screen.
- We have also developed a SPR-assisted fragment-based screening method with necessary sensitivity and throughput in which we can perform complete screens on libraries of several thousand compounds in just a few weeks per target.
Targets identification
Combing the SPR assays and specialized knowledge in proteomics, our team is able to determine the nature of protein complexes and their function, investigate the binding properties of protein isofoms and identify binding partners to any target molecules-ligand fishing.
Target and assay validation
- Rapid classification of the mechanism of action of any inhibitor, agonist or other ligand can be achieved.
- A series of accurate characterization parameters, such as complex formation (ka), complex stability (kd) and affinities (KD) are obtained from SPR, and can be used in simultaneous identification of targets.
- We are also capable of quickly establishing high throughput binding screening methods for the validation for both target and assay process.
Hit to lead characterization
- SPR biosensor enables us to measure membrane protein-ligand binding interactions, most importantly having these classes of membrane protein in native lipid states that retains its native structure and activity.
- Thanks to its unique label-free property, we can measure real-time binding interaction.
- We can obtain the important information of quantitative affinity ranking and detailed kinetics of interaction for small molecules binding to target proteins.
- Comprehensive studies in hit to lead characterization can be achieved with the use of SPR technique.
Lead optimization
- We use SPR to prioritize inhibitors, agonists or ligands for lead compound development and study QSAR-based kinetic evaluation of a lead compound, guaranteeing the correct selection of compounds on conditions that are related to target binding and target selectivity in vivo.
- In addition, we can also apply SPR in rapid in vitro ADME assays for characterization of therapeutic lead compounds and adjustment in time.
Our Capabilities of SPR
- Our experienced experts provide dynamic analysis of hundreds of molecular interactions, which can be performed simultaneously in our system.
- The probability of false positive molecules due to nonspecific binding will be greatly reduced in screening of drug candidates through the complete collection of high resolution binding and accurate analysis of characterization information.
Equipments
Open SPR facilities guarantee efficient and accurate data generation with maximized throughput:
Biacore T200.
Reference
- Patching, S. G. Surface plasmon resonance spectroscopy for characterisation of membrane protein-ligand interactions and its potential for drug discovery. Biochimica Et Biophysica Acta. 2014, 1838(1): 43-55.
※ It should be noted that our service is only used for research.

One-stop
Drug Discovery Services
- Experienced and qualified scientists functioning as project managers or study director
- Independent quality unit assuring regulatory compliance
- Methods validated per ICH GLP/GMP guidelines
- Rigorous sample tracking and handling procedures to prevent mistakes
- Controlled laboratory environment to prevent a whole new level of success
Online Inquiry

