LC-MS for Target Separation and Detection
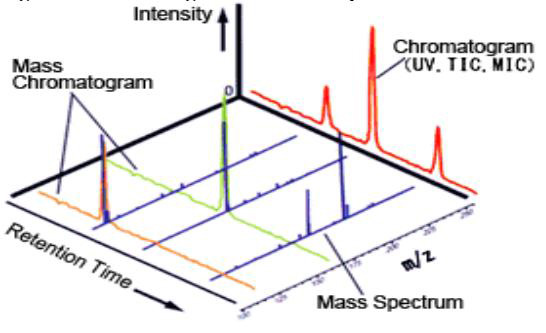
Fig.1 Chromatogram and Mass Spectrum. (Sarvani, V.; et al. 2013)
Liquid Chromatography-Mass Spectrometry (LC-MS)
Liquid chromatography-mass spectrometry (LC-MS) has developed to become a powerful, indisposable and essential bioanalytical technology. A high-throughput LC-MS process require conditions for separation and detection of excellent reproducibility, robustness, and low fault liability. Early drug discovery projects make full use of LC-MS screening approaches which selectively detect chemical functionalities of target molecules. Liquid chromatography conduct safe separation of a wide range of organic compounds from small-molecule drug metabolites to peptides and proteins. Chromatographic process is therefore applied as a separation technique in mass spectrometry helping in both qualitative and quantitative analysis of candidate drugs.
Advantages of LC -MS
LC-MS has several advantages over MS and they can be summarized as the following aspects:
- A large numbers of drugs can be identified in just one condensed analysis window.
- As a presumptive method, LC-MS greatly reduces the use of reagent which is cost-effective for the drug discovery project.
- LC-MS can rapidly generate better data quality and is easy to operate.
Process of LC -MS
The whole process of LC-MS can involve the following three steps:
- Separate mixtures in accordance with their physical and chemical properties.
- Identify the components within each peak.
- Detect each of them on the basis of their mass spectrum.
LC -MS Services
We have adapted LC-MS in different stages of drug discovery and provided multiply services to meet your demand.
- Determination of the molecular weight with the support of LC-MS for analysis of lead candidate.
- Protein identification and metabolite stability profile using LC-MS in the lead candidate validation.
- Confirmation of peptide and glycoprotein identification in target identification.
- Bio-affinity screening analysis is performed with LC-MS in lead identification.
- Pharmacokinetic screening and metabolic stability analysis in the lead optimization.
Equipment
Agilent 6550 iFunnel Q-TOF LC-MS
Reference
- Sarvani, V.; et al. Role of LC-MS in drug dicovery process. International Journal of Pharmacy & Therapeutics. 2013, 4(3): 148-153.
※ It should be noted that our service is only used for research.

One-stop
Drug Discovery Services
- Experienced and qualified scientists functioning as project managers or study director
- Independent quality unit assuring regulatory compliance
- Methods validated per ICH GLP/GMP guidelines
- Rigorous sample tracking and handling procedures to prevent mistakes
- Controlled laboratory environment to prevent a whole new level of success
Online Inquiry

